| January 16. 2021
Ellie Burleigh (MPH, Phd)
This update includes new information and guidance from US and Mexican sources regarding vaccine approvals, vaccine availability, answers to FAQs, descriptions of vaccine products and their distribution, and plans for additional vaccines.
Which vaccines have been approved in the US and Mexico so far?
US: Two vaccines have been approved for emergency use in the United States: The Pfizer-BioNTech vaccine and the Moderna vaccine. Both are mRNA vaccines. Both require two doses, spaced three or four weeks apart. The first dose, the primary dose, provides some immunity during the time between the two doses but it is the second dose that raises immunity levels to an astonishing 94-95%.
Mexico: To date, Mexico has approved the Pfizer-BioNTech vaccine and the AstraZeneca vaccine (https://elpais.com/mexico/2021-01-05/mexico-aprueba-la-vacuna-contra-el-coronavirus-de-oxford-y-astrazeneca.html). The Pfizer-BioNTech vaccine is an mRNA vaccine. The AstraZeneca vaccine is not. It is an adeno-virus vectored vaccine. Both of these vaccines require two doses, spaced about a month apart. Detail on each of the approved vaccines is provided be low.
These are the only vaccines approved in each country so far. In spite of rumors of other vaccine sources, Mexico has not approved any other vaccine to date.
Vaccination Phases
Mexico: In Phase I: Vaccination of health personnel – January, 2021
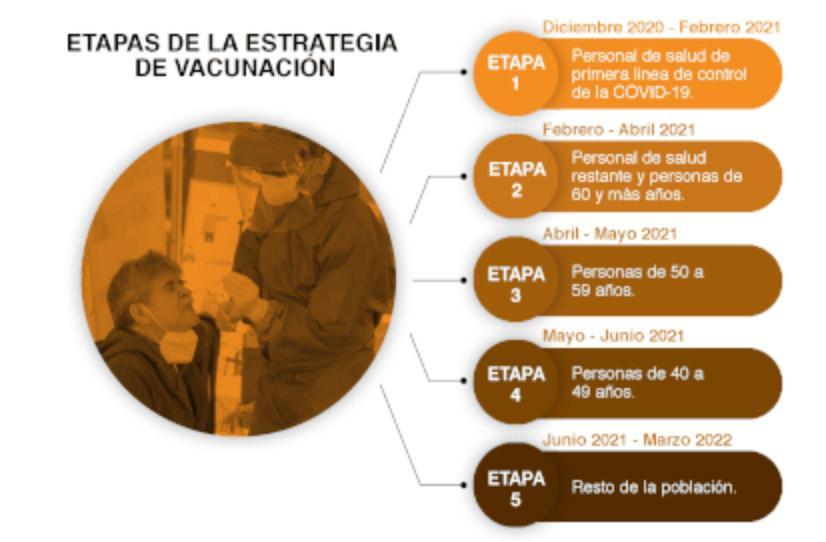
begin vaccinating people against COVID-19 nationally. On that same date, shipments began arriving of over 400,000 doses of the Pfizer-BioNTech vaccine. The objective is to have vaccinated all medical and health personnel who work directly with persons with COVID-19 in the 1,015 “COVID-19 hospitals” in the country by the end of January. Each person is to receive the recommended two shots 3 weeks apart.
https://www.infobae.com/america/mexico/2021/01/06/calendario-de-vacunacion-covid-19-en-mexico-cuando-me-toca-de-acuerdo-a-mi-edad/
The next step beginning in February will be to vaccinate the elderly, beginning with those over 80, 70-79, 60-69, etc., until all those over 60 are vaccinated. Dr. Lopez-Gatell, SubSecretary of Health, has been clear that brigades will begin working first in the most remote areas of the country once they have completed the vaccination of all health personnel.
On January 6, Dr. Lopez Gatell, explained that vaccinations for the elderly will be given by brigades made up of trained public servants and volunteers. Each brigade will be set up in one of 10,000 Integrated Centers, to be located in the geographical center of 280,000 small, disperse communities in the country. Centers will be located where the elderly are accustomed to receive their pensions (a school, rural medical unit, a plaza, etc.). Each Center will vaccinate 300 elderly adults each week. In this way, Mexico will immunize a total of 3 million elderly persons in remote areas. Once the Centers are installed, the persons will receive their vaccine when they receive their bi-monthly pension. If the person does not come, a brigadista will go to their home. A group of authorities from the communities surrounding each Center will send a report of the vaccination campaign to the capital of each state.
Following this, vaccinations will proceed in the 2,500 municipalities or municipal heads, and then the largest cities. When the same brigades reach the large cities, they will work together, with each brigade responsible for vaccinating 300 persons a week. Once all of the elderly are vaccinated, vaccination will continue with persons under 60 who have chronic diseases, and with teachers under 60 years of age.
Guanajuato
On January 11, 2021, The Secretary of Health of Guanajuato announced that the State is ready to begin vaccinating its 22,425 health professionals against COVID-19. It will take up to 18 months to immunize over 70% of the population of Guanajuato. Guanajuato has the ability to vaccinate large numbers of people, and will follow the national plan. https://boletines.guanajuato.gob.mx/2021/01/11/guanajuato-esta-preparado-para-iniciar-este-miercoles-la-vacunacion-contra-el-coronavirus/
Those excluded will include children under 16, and pregnant women.
Plans for additional vaccines
Mexico
Information about the vaccine portfolio published in the National Vaccination Program is as follows:
- Pfizer-BioNTech. – to immunize 17.2 m people, arrival Dec. 20
- AstraZeneca – to immunize 38.7 m people, arrival March 21
- CanSino Biologica- to immunize 35 m people, arrival Feb 21
- Sputnik V- to immunize 12 m people, arrive late Jan 21 (delayed)
Four offers for other vaccines are being considered for approval by the Mexican government
- Moderna COVID-19 vaccine
- Novavax COVID-19 vaccine
- Janssen/Johnson and Johnson’s COVID-19 vaccine
- CureVac COVID-19 vaccine
USA
The US has given Emergency approval (EUA) to the Pfizer and the Moderna vaccines and has begun vaccination in all states. The vaccination situation for each state can be found online. According to CDC, as of January 11, 2021, nearly 9 million initial doses of the two approved vaccines have been administered. Each state is administering its own program. If you decide to get vaccinated in the US, check your state’s guidance, and register if possible.
The problems with the US vaccination roll-out have been due, primarily, to a confusion in lines of command and responsibility. Initially, states were led to believe that the federal government would be coordinating the entire program, with the help of the military. Later, this proved not to be the case, and the responsibility was unexpectedly given to each of the states. Very few had prepared the necessary personnel and cold chain to handle a massive vaccination campaign. Most states have tried to rise to the challenge, developing plans and approaches, but many still struggle. Hopefully this will straighten itself out soon.
Additional COVID-19 vaccines are being considered in the US and expected to be submitted to the FDA for EUA approval in 2021. Large-scale (Phase 3) clinical trials are in progress for additional COVID-19 vaccines in the US.
- Janssen/Johnson and Johnson’s COVID-19 vaccine
- AstraZeneca
Answers to a few questions (FAQ’S)
Questions and answers from the previous update are included in this one so that readers don’t have to look back to find them. If there have been no changes in the answers, they have been moved to the bottom. New questions or questions with updated answers are near the top.
If I live in Mexico legally, but am not Mexican, can I get vaccinated here?
On Jan 8, 2021, the Mexican Government announced that Mexico will vaccinate foreigners residing in Mexico free of charge. This was in response to threats from the US that undocumented Mexican workers in the US might not be eligible to receive the vaccine. Mexico stated its expectation of reciprocity in the treatment of its citizens abroad in the future.
If I am a foreigner living in Mexico and could get vaccinated elsewhere, should I stay and get vaccinated in Mexico?
As everyone’s situation is different, this is an individual decision. Here are some facts to consider: The vaccines are the same product whether you get them here in Mexico or elsewhere. The quality of the product at the site of manufacture and the guidelines about taking them are the same. You must get two doses of the same product 3-4 weeks apart. Doses from different products cannot be used.
One of the vaccines approved in Mexico to date, the Pfizer-BioNTech vaccine, has been approved by the FDA so far. The AstraZeneca vaccine approved in Mexico is not yet being considered by the FDA. It must go through additional Phase 3 testing before it may be considered for approval.
The Pfizer vaccine has a difficult storage and distribution requirement, which makes it challenging to protect along the cold chain from the manufacturer to your arm. It must be kept at really cold temperatures or it runs the risk of becoming ineffective before you get it. This need for ultra-refrigeration will be a challenge in the US, but will pose a significant challenge in Mexico. Even ensuring a vaccine has a secure standard refrigeration cold chain, required by AstraZeneca, will pose a significant challenge in Mexico, particularly in rural areas.
For these reasons, Be Well San Miguel has recommended receiving your vaccine in the US, if at all possible. This will also reduce the demand for vaccines on the reduced Mexican supply.
How do I take the second dose of the mRNA vaccines?
The following recommendations are from the recent CDC update, Jan 6, 2021. Although AstraZeneca has not yet been approved in the US. I have included information about it below.
The mRNA COVID-19 vaccine series consist of two doses administered intramuscularly:
- Pfizer-BioNTech: 3 weeks (21 days) apart
- Moderna: 1 month (28 days) apartAstraZenica is also a series of two doses administered intramuscularly 1 month (28 days) apart
Persons should not be scheduled to receive the second dose earlier than recommended. The vaccine products are not interchangeable with each other or with other COVID-19 vaccine products. The safety and efficacy of a mixed-product series have not been evaluated. Both doses of the series should be completed with the same product. The need for and timing of booster doses for vaccines has not been established. No additional doses beyond the two-dose primary series are recommended at this time.
Should I get vaccinated if I think I have been exposed to someone with COVID-19?
Persons in the community or outpatient setting who have had a known COVID-19 exposure should not seek vaccination until their quarantine period has ended to avoid potentially exposing healthcare personnel and other persons to SARS-CoV-2 during the vaccination visit.”
What counts as exposure to someone with COVID-19?
- You were within 6 feet of someone who has COVID-19 for a total of 15 minutes or more with or without a mask
- You provided care at home to someone who is sick with COVID-19
- You had direct physical contact with the person (hugged or kissed them)
- You shared eating or drinking utensils
- They sneezed, coughed, or somehow got respiratory droplets on you
If you think you have been exposed, you need to:
- Stay home and monitor your health
- Stay home for 14 days after your last contact with a person who has COVID-19.
- Watch for fever (100.4◦F), cough, shortness of breath, or other symptoms of COVID-19
- If possible, stay away from others, especially people who are at higher risk for getting very sick from COVID-19
Should I get vaccinated if I have an HIV infection, other immunocompromising condition or take immunosuppressive medications or therapies?
The most recent CDC guidance from January 6, 2021 says that persons with HIV infection or other immunocompromising conditions, or who take immunosuppressive medications or therapies might be at increased risk for severe COVID-19, however data are not currently available to establish vaccine safety and efficacy in these groups. Persons with stable HIV infection were included in mRNA COVID-19 vaccine clinical trials, though data remain limited. No imbalances were observed in the occurrence of symptoms consistent with autoimmune conditions or inflammatory disorders in clinical trial participants who received an mRNA COVID-19 vaccine compared to placebo. Persons with autoimmune conditions who have no contraindications to vaccination may receive an mRNA COVID-19 vaccine. However, they should be counseled about the unknown vaccine safety profile and effectiveness in immunocompromised populations, as well as the potential for reduced immune responses.
Persons with a history of Guillain-Barré syndrome, a history of Bell’s palsy, Allergic reactions
According to CDC, to date, no cases of Guillain-Barré syndrome (GBS) have been reported following vaccination in the Pfizer-BioNTech or Moderna COVID-19 vaccines clinical trials. Persons with a history of GBS may receive an mRNA COVID-19 vaccine unless they have a contraindication to vaccination. Cases of Bell’s palsy were reported following vaccination in both the Pfizer-BioNTech and Moderna COVID-19 vaccines clinical trials. However, the FDA does not consider these to be above the frequency expected in the general population and has not concluded that these cases were causally related to vaccination. A handful of people receiving the Pfizer vaccine have had allergic reactions. If you are allergic to any of the ingredients in the vaccines, you should discuss receiving the vaccine with your doctor. EpiPens are being made available at all vaccination sites in case of a reaction. Studies with these groups are now underway.
Should pregnant women get vaccinated?
Mexico
For those of you counseling Mexican friends or colleagues, the Mexican Ministry of Health does not plan to vaccinate pregnant women. Pregnant women were not included in Phase 3 trials for Pfizer or AstraZeneca.
US
The safety of pregnant women, their fetuses and breastfeeding women were also not included in vaccine Phase three trails for Moderna. For this reason, the initial FDA EUA (and our initial update) recommended that these groups not receive the vaccines. This recommendation has been updated by the CDC (with links below) as follows:
“Getting vaccinated is a personal choice for women who are pregnant. If they also belong to a group recommended to receive a vaccine, they may choose to be vaccinated. If they have questions about getting vaccinated, a discussion with a healthcare provider might help them make an informed decision. While a conversation with a healthcare provider may be helpful, it is not required prior to vaccination. Observational data demonstrate that, while the chances for these severe health effects are low, pregnant women with COVID-19 have an increased risk of severe illness, including illness that results in ICU admission, mechanical ventilation, and death compared with non-pregnant women of reproductive age. Additionally, women with COVID-19 might be at increased risk of adverse pregnancy outcomes, such as preterm birth, compared with pregnant women without COVID-19. Until findings are available from clinical trials and additional studies, only limited data are available on the safety of COVID-19 vaccines, including mRNA vaccines, administered during pregnancy:
- Limited data are currently available from animal developmental and reproductive toxicity studies. No safety concerns were demonstrated in rats that received Moderna COVID-19 vaccine before or during pregnancy; studies of the Pfizer-BioNTech vaccine are ongoing.
- Studies in people who are pregnant are planned.
- Both vaccine manufacturers are monitoring people in the clinical trials who became pregnant.
- mRNA vaccines do not contain the live virus that causes COVID-19 and, therefore, cannot give someone COVID-19. Additionally, mRNA vaccines do not interact with a person’s DNA because the mRNA does not enter the nucleus of the cell. Cells break down the mRNA quickly. Based on how mRNA vaccines work, experts believe they are unlikely to pose a specific risk for people who are pregnant. However, the actual risks of mRNA vaccines to the pregnant woman and her fetus are unknown because these vaccines have not been studied in pregnant women.
Routine testing for pregnancy before COVID-19 vaccination is not recommended. Women who are trying to become pregnant do not need to avoid pregnancy after receiving an mRNA COVID-19 vaccine.
Breastfeeding women or their infants?
According to DC, there are no data on the safety of COVID-19 vaccines in lactating women or on the effects of Pfizer or Moderna vaccines on the breastfed infant or on milk production/excretion. mRNA vaccines are not thought to be a risk to the breastfeeding infant. If women who are breastfeeding are part of a group recommended to receive a COVID-19 vaccine, such as healthcare personnel, they may choose to be vaccinated.
Additional Guidance for pregnant or lactating women
Additional guidance may be found at the American College of Obstetricians and Gynecologists, https://www.acog.org/ and the Society for Maternal Fetal Medicine, https://www.smfm.org/.
Once vaccinated am I risk free from getting the COVID-19 virus?
Vaccines provide varying levels of immunity for YOU but not for those around you. According to the CDC, neither Pfizer nor Moderna has yet proven to keep you from being infected with COVID-19 but rather keeps you from getting sick from the disease. However, if you are vaccinated and then become exposed to COVID-19, you may still be infectious even if you have no symptoms. Until everyone has been vaccinated you should still take precautions not to infect others by wearing masks, keeping safe distances, and washing hands. Studies on how infectious a person is once vaccinated are ongoing so keep an eye out for developing news.
Should I get a vaccination if I know or think that I have already been infected with COVID-19?
The CDC recommendation is that you SHOULD get vaccinated. The new CDC guidance as of January 6, 2021 says vaccination should be offered to persons regardless of history of prior symptomatic or asymptomatic SARS-CoV-2 infection. Viral testing to assess for acute SARS-CoV-2 infection or serologic testing to assess for prior infection solely for the purposes of vaccine decision-making is not recommended.
Should I get a vaccination if I am currently sick with Covid-19?
CDC Jan 6 2021 guidance says that vaccination of persons with known current SARS-CoV-2 infection should be deferred until the person has recovered from the acute illness (if the person had symptoms) and criteria have been met for them to discontinue isolation. This recommendation applies to persons who develop SARS-CoV-2 infection before receiving any vaccine doses as well as those who develop SARS-CoV-2 infection after the first dose but before receipt of the second dose. While there is otherwise no recommended minimum interval between infection and vaccination, current evidence suggests that reinfection is uncommon in the 90 days after initial infection. Thus, persons with documented acute SARS-CoV-2 infection in the preceding 90 days may delay vaccination until near the end of this period, if desired.
If I am vaccinated and then get COVID-19, does that affect my treatment?
For vaccinated persons who subsequently develop COVID-19, the vaccine should not affect treatment decisions (including use of monoclonal antibodies, convalescent plasma, antiviral treatment, or corticosteroid administration) or timing of such treatments.
Should children under 18 and under 16 get vaccinated?
Under the EUAs, the following age groups are authorized to receive vaccination:
- Pfizer-BioNTech: ages 16 or over
- Moderna: ages 18 or over
- AstraZeneca: Ages 16 or over (Mexico)
Children and adolescents outside of these authorized age groups should not receive COVID-19 vaccination at this time.
When can life goes back to normal?
Until we reach population or herd immunity which is 70% of the population immune through vaccination or through previous infection with Covid-19, we cannot go completely back to life as we knew it. This is why it is so important that all of us to get the vaccination (except those contra-indicated by each vaccine, above). Once we reach this percentage, the virus does not have enough victims left to infect and it will lose its power. Life as we knew it can resume safely. This is the goal.

Detail on the three approved vaccines
What kind of vaccines are these and what do they do? Can they give you COVID-19? Can they affect your DNA?
The SARS-CoV-2 virus is studded with proteins that it uses to enter human cells. These so-called spike proteins make a tempting target for potential vaccines and treatments.
Both the Pfizer and Moderna vaccines are mRNA (messenger ribonucleic acid) vaccines. The AstraZeneca vaccine is made differently. It is a viral-vector vaccine. Unlike the Pfizer-BioNTech and Moderna vaccines, which store the instructions for the spike protein in single-stranded RNA, the AstraZeneca vaccine uses double-stranded DNA. The DNA for the spike protein is added to another virus called an adenovirus. Adenoviruses are common viruses that typically cause colds or flu-like symptoms. The Oxford-AstraZeneca team used a modified version of a chimpanzee adenovirus that causes colds in chimpanzees (not humans). It can enter cells, but it can’t replicate inside them.
None of these vaccines can give you COVID-19 or affect your DNA. All three tell your cells to make a small harmless copy of the spike protein unique to COVID-19. Your body then quickly builds a strong natural immune response. That strong natural immune response remains in your body to protect you from the virus if you get infected.
Are the vaccines safe?
All three vaccines have gone through three phase trials. This is required by both the US and Mexico. Given the seriousness of the COVID-19 virus and its virulence, the third phase trial requirement has been shortened for emergency approval in both countries. If no significant negative impact on human subjects is detected during the third phase, the vaccine can be considered for emergency approval.
Phase three of the Pfizer vaccine randomly assigned 37,000 people to receive either two shots of the vaccine or two saline shots (placebo). They waited for 7 days after the second shot for the immune response to occur and then counted any COVID-19 cases that occurred 7 days later in both groups. Out of the 170 cases that occurred, only 8 were in the group vaccinated and the rest were in the group given the placebo. Four cases of Bells Palsy occurred, and an allergic reaction in 3 individuals within around 10 minutes after they were given the shot. These were mainly in those who have had prior allergic reactions to other drugs, but not all. Otherwise, the only negative reactions are fatigue and headaches after the second dose, pain at the injection site and muscle pain. To date, the occurrence of negative reactions has not been frequent or serious enough to prevent approval or take the vaccine off of the market.
Phase three of the Moderna vaccine randomly assigned 30,000 people to receive either two shots of the vaccine given four weeks apart or two saline shots (placebo). They then waited for two weeks for the immune response to respond, and then counted any COVID-19 cases that occurred during the next month. Out of the 196 cases that occurred that month, only 11 were in the group that was vaccinated. Three of the 15,000 people who got the vaccine got temporary Bells palsy, as did one person out of the 15,000 people who got the placebo. Some people got lymph node swelling after the second dose, but no one had an allergic reaction. Reactions similar to the Pfizer vaccine occurred after the shot was given. No negative reactions were considered serious or common enough to prevent the Moderna vaccine from approval.
AstraZeneca: The University of Oxford partnered with the British-Swedish company AstraZeneca to develop and test the AstraZeneca vaccine. The Phase three trial was conducted in the UK and Brazil, and results were pooled. Results revealed that the vaccine was 62 to 90 percent effective, depending on the initial dosage. In one trial, participants were accidentally given half a dose of the vaccine followed a month later by a full dose. Surprisingly, the vaccine combination in which the first dose was only half strength was 90 percent effective at preventing Covid-19 in the clinical trial. In contrast, in another trial the combination of two full-dose shots led to just 62 percent efficacy. The average efficacy, the developers said, was 70 percent. Almost immediately, though, there were doubts about the data. AstraZeneca disclosed in its initial announcement that fewer than 2,800 participants received the smaller dosing regimen, compared with nearly 8,900 participants who received two full doses. The biggest questions were, why was there such a large variation in the effectiveness of the vaccine at different doses, and why did a smaller dose appear to produce much better results? AstraZeneca and Oxford researchers said they did not know. Crucial information was also missing. The company said that the early analysis was based on 131 symptomatic Covid-19 cases that had turned up in study participants. But it did not break down how many cases were found in each group of participants — those who received the half-strength initial dose, the regular-strength initial dose and the placebo. The UK and Mexico have approved the AstraZeneca vaccine, nonetheless. The US has requested additional trials before the vaccine can be considered for approval.
What is the Cold Chain and why is it important?
The Cold Chain refers to the chain of different cold storage devices (cold containers, refrigerators, ice chests, thermoses, vials, etc.) that are critically needed to maintain a vaccine at its required temperature as it moves on its journey from the manufacturer to the moment it is injected into people’s arms.
Approved COVID-19 vaccines will be flown to Mexico and the various US states from the manufacturer at critically defined temperatures, then moved quickly to their destinations. If at any point during the chain (air-cargo, trucking, hospital storage, transfer to remote clinics, etc.) the vaccine deviates from its required temperature, the vaccine will expire and cannot be used. As you can imagine, unintentional breaks in the chain could happen any place along the journey from the manufacturing plant to your arm. Breaks in the Cold Chain result every year to in a loss of many different types of vaccines. Although this can happen anywhere, in any country, the cold chain is especially challenging in countries like Mexico, with scarce resources and disperse rural communities.
Having said that, however, with assistance from the WHO, the Pan-American Health Organization and UNICEF, developing countries often have more experience than developed countries in mounting large-scale vaccination campaigns. The brigade concept being put into practice by the Mexican government for the vaccination of the elderly could be an example of that.
The Pfizer vaccine is especially tricky since it must be kept at a continuous -94F (-70C) from the manufacturer to the place where it will be stored – usually a hospital that is capable of storing vaccines at extremely low temperatures. The State of Guanajuato has said it is confident it will be able to ensure the cold-chain for the Pfizer vaccine. The Policy states that the Pfizer vaccine will be kept at -70C in super-freezers and thawed before use, applying 975 doses in five days, each vial of five doses to be applied in less than six hours so that the vaccine stays intact.
The Moderna vaccine cold chain will be easier to handle though still challenging for some more rural conditions. Moderna’s vaccine has to be shipped at –20 degrees Celsius (–4 degrees Fahrenheit), and it can then be stored at that temperature for six months. Once thawed, it can be kept in a refrigerator between two and eight degrees C (36 to 46 degrees F) for up to 30 days. This is within the range of a normal refrigerator.
The AstraZeneca vaccine cold chain will also be easier to handle as long as there is refrigeration. The AstraZeneca vaccine for Covid-19 is more rugged than the mRNA vaccines from Pfizer and Moderna. DNA is not as fragile as RNA, and the adenovirus’s tough protein coat helps protect the genetic material inside. As a result, the AstraZeneca vaccine doesn’t have to stay frozen. The vaccine is expected to last for at least six months when refrigerated between two and eight degrees C (38–46°F).
And that’s it for now! I hope this has been useful.
Ellie Burleigh (MPH, Phd) |