| By Deborah Bickel PA MPH
This is part of a series of articles warning our clients about very common, drugs, environmental pollution and other current changes in medical opinion that may affect how you manage your minor to major illnesses. The first newsletter in the series was about Fluoroquinolones. I was shocked by how many people wrote to say they had had common side effects to the antibiotic, including ruptured tendons. A few of the respondents told their physicians about the relationship and the most common response was “I have never heard of that.’ I encourage all of you to share this information with your physicians. I give sources for the information I am sharing, and they are all reputable.
This newsletter is about Zantac, known as Ranitidine in generic form.
Everyone who takes pharmaceuticals drugs has a favorite one they need often. Mine was Ranitidine or Zantac. I often have an acidic stomach and it helped every time. Imagine my dismay when information began coming out that Ranitidine “contained” a carcinogen. I immediately began my research, which I will share with you. It is always a good idea to take as few drugs as you can and hope for the best.
Ranitidine is available over the counter in Mexico and most other countries. It is the one of the most common drugs used for heartburn or other gastrointestinal problems such as gastrointestinal reflux (GERD). It is made by a French company and distributed in the US , Canada and Mexico by Norvartis / Sandoz. When the warning first came out in September of this year, Ranitidine was believed to contain a cancer-causing contaminant in the generic forms. This has proven to not be the case. The chemical is actually a common breakdown product found in many foods as well as in drinking water. Because we ingest this carcinogen in tiny amounts, it has not been targeted as a major cancer-causing agent. In larger doses taken frequently (such as with chronic use of ranitidine) it can be implicated in causing many common cancers.
Remember: “cancer causing” means very little if it increases the odds only fractionally. We are constantly bombarded with potential cancer-causing agents. Our bodies are resilient thank God. But here is the story:
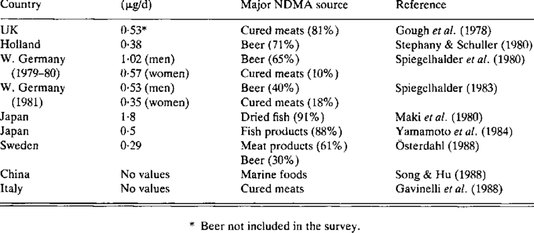
The breakdown product is NDMA or N-nitroso dimethylamine). The Federal Drug Administration did not identify the problem but rather a small reputable online pharmacy called Valisure. Their Stanford founders test drugs commonly used in the US because they think that many drugs might not be safe despite FDA approval. Its scientists alerted regulators that Zantac and Ranitidine contain NDMA. Despite the fact that the warning was issued in the US by a small pharmaceutical lab, more than 40 countries, both in the developed and developing world, immediately stopped sales, started their own investigations, or took steps to protect consumers. Mexico was not initially one of those countries, but may have just started pulling the drug. More than a month later, the Food and Drug Administration confirmed unacceptable levels of NDMA in some ranitidine products — including in syrups given to infants.
 Unsafe storage and handling (especially by the time it reaches Mexico!) Unsafe storage and handling (especially by the time it reaches Mexico!)
At first NDMA was thought to be a contaminant only found in generic forms of the drug, but it was soon discovered to be a naturally occurring breakdown product that released dangerous amounts of the chemical under extreme heat. Keep in mind that many drugs suffer overheating in transport and storage. Those of you who receive drugs from the US should be aware that all drugs unless refrigerated are exposed to heat from 120 to 149 F when sitting at a hot border or waiting for unloading. This is nowhere near as high as the 175-degree heat applied in testing, but it is still a problem because the relatively lower heat encountered in transport is still associated with some release of NDMA.
What to Do ?
The obvious solution to the problem is to stop taking ranitidine unless it is for a very short course, such as two to three weeks. The other drugs in the same category or used for the same gastrointestinal conditions have not been found to be dangerous. Ranitidine is in the category of drugs called H2 inhibitors. Another drug class used for similar purposes is the widely available protein pump inhibitor or PPI. One of the most common PPIs is Omeprazole.
Safer Drugs for stomach acid ?
The drugs in a similar category as ranitidine (H2 blockers) not found to release NDMA are:
– Famotidine or Pepcid and
– Cimetidine or Tagamet
Note: Tagamet interferes with many drugs. See https://www.drugs.com/drug-interactions/cimetidine,tagamet-hb.html for a full list. The more common drugs are theophylline, blood thinners, and Erythromycin related drugs. In combinations and even alone, Tagamet can be toxic for some patients mostly to the liver and kidneys.
Protein pump inhibitors are also problematic as they interact with other drugs as well. The most important and probably most common drug in SMA is the anticoagulant Plavix 9, generically known as Clopidogrel.
Ranitidine in disguise
BEWARE! Ranitidine is still being sold in pharmacies here in town. The names of the product could be deceptive. A few names are Ulgastrin, Azantac, and Geldose. I found it named Pepmo in one popular pharmacy – an obvious attempt to disguise Ranitidine as Pepsid which is a safer and equally effective alternative. The people working behind the counter at most pharmacies seldom know what brand names contain what generics. The names of drugs change rapidly and often are so similar to other drugs that mistakes can be easily made by both the consumer and the salesperson.
COPRAPRIS (Mexico’s equivalent to the FDA) has asked physicians to not prescribe Ranitidine, but it was still widely available without a prescription two weeks ago. The other day I asked at Similares about it, and they said it has been pulled off the shelves.
There is a wealth of non-pharmaceutical actions that can keep GIRD and hyperacidity at bay. A great site covering those interventions is https://www.health.harvard.edu/digestive-health/9-ways-to-relieve-acid-reflux-without-medications
One easy and surprisingly helpful method is to chew gum and of course to avoid high acid foods and not g too long between meals. Some people might find antacids useful for relieving heartburn. Lifestyle changes, including avoiding certain foods and beverages, such as spicy foods, large or fatty meals, and alcohol, can also help prevent episodes of heartburn.
As FDA and other agencies around the world continue to investigate Ranitidine, more details will become available. In the meantime, the FDA is not calling for individuals to stop taking the medication.
Here is a quote from the FDA: “Valisure is working with researchers at Memorial Sloan Kettering Cancer Center to dig deeper into the risks. They hope to be able to pinpoint which types of cancer are linked to NDMA soon, so people who’ve been taking ranitidine for years will know what to monitor with their doctors”.
However, for many conditions, Ranitidine is only recommended for short-term use. If you have been using it for a while, now would be a good time to discuss with your physician whether you still need it, and whether you might benefit from alternative treatment options, including other drug classes or a different H2 blocker. Based on what is known so far, there is no evidence that other H2 blockers or other heartburn medications are affected by NDMA impurities.
Cardiac medications that produce NDMA
Finally and very importantly, another drug class that many cardiac patients are on has been recalled due to NDMA production. These medications are called Angiotensin II Receptor Blockers (ARBs), and are used to treat blood pressure issues and heart failure. The agency has issued numerous recalls for NDMA contaminated ARBs including Valsartan, Diovan, Losartan, and Irbesartan. Speak to your cardiologist about the risks and benefits before stopping any of these medications.
As for being deprived of my favorite drug, I am drinking water instead and finding it actually seems to help more than 50% of the time. Probably time to rethink my go to medications and try for non-pharmaceutical alternatives!
Some of the links to reviews and studies informing this article
https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine
https://www.scientificamerican.com › article › what-we-know-about-the-po…
https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and |